Lithium-Sulfur Batteries: Unlocking Longer EV Range?
Let’s be honest: when you think about buying an electric car, you probably ask yourself, “How far can it go on a single charge?” This concern is often referred to as range anxiety—that small worry about whether you’ll reach your destination or the next charging station. Today’s electric cars are impressive; however, their lithium-ion batteries have limitations. They only have so much energy they can store before they become too heavy or expensive.
But what if a new battery technology could offer more energy in a lighter, more affordable package? Here’s where lithium-sulfur (Li-S) batteries come in. Many experts are calling them the next big breakthrough in battery technology. They can dramatically enhance the driving range of electric automobiles.
In this article, we’ll explain what Lithium-Sulfur batteries are and how they work, in simple terms. We’ll also discuss why they hold such promise for extending the range of your electric car.
What Are Lithium-Sulfur Batteries And How Do They Work?
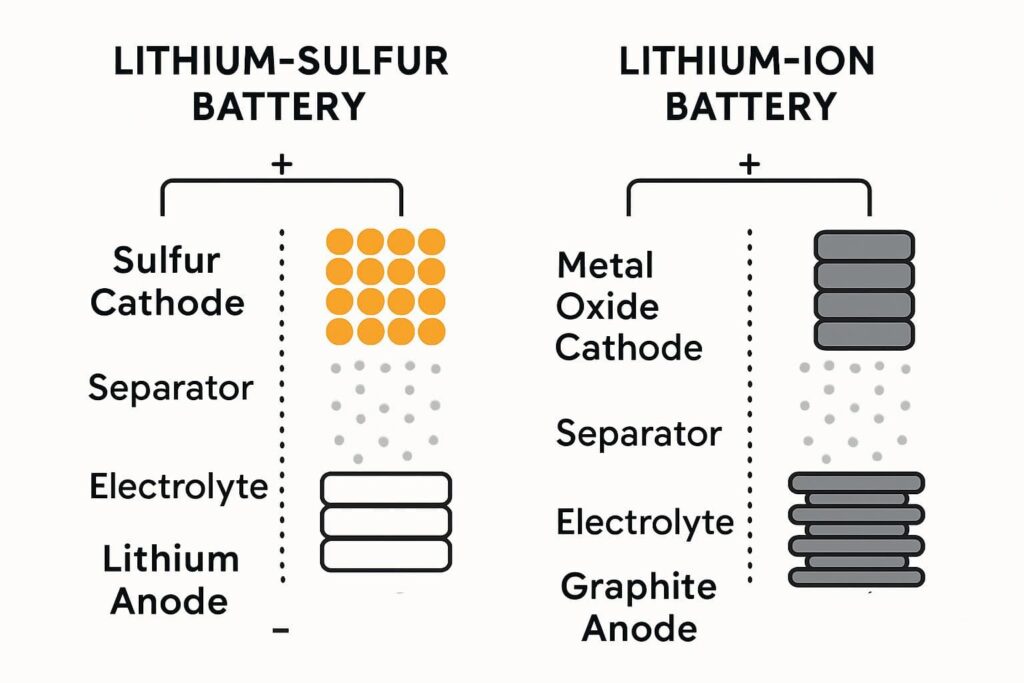
Lithium-sulfur batteries are rechargeable batteries. They are similar to lithium-ion batteries seen in smartphones and electric vehicles. But they use different materials. This difference creates both exciting possibilities and challenges.
Instead of graphite for the negative electrode (anode) and metal oxides for the positive electrode (cathode), Lithium-Sulfur batteries use simpler, lighter materials:
Anode (Negative Electrode): This is pure lithium metal. It is extremely light and stores a large amount of energy.
Cathode (Positive Electrode): Elemental sulfur, a lightweight and common yellow element, serves as the cathode due to its high energy storage capacity.
How They Make Electricity
Imagine tiny charged particles called lithium ions (Li+). When you utilize the battery (discharge), these lithium ions leave the lithium metal anode. They travel through a liquid known as an electrolyte and then to the sulfur cathode.
When they reach the cathode, they react with the sulfur. This forms different compounds called lithium sulfides (like Li2S). This chemical reaction releases electrons. These electrons generate the electrical current that powers your electric vehicle.
When you charge the battery, the process reverses. Electricity causes lithium ions to escape the sulfur cathode. They travel back through the electrolyte and attach back to the lithium metal anode. The battery is now ready for its next discharge.
Sounds simple, right? Well, a key part of the process involves those intermediate lithium sulfide compounds formed at the cathode. These are often called lithium polysulfides (Li2Sx). Consider them as sulfur chains of varying lengths with lithium connected. These polysulfides have a tendency to dissolve into the electrolyte, which, as we’ll see later, causes some problems. But first, let’s focus on the exciting potential.
Why Li-S Could Revolutionize EV Range
Researchers and car manufacturers are excited about Lithium-Sulfur (Li-S) technology. This technology could lead to better electric vehicles (EVs) for you in the future.
Advantage 1: Lots of Stored Energy
This is the most impressive benefit. “Energy density” refers to how much energy a battery can hold for its weight. This is measured in Watt-hours per kilogram. A higher number means a better battery.
Lithium-sulfur batteries could store much energy, potentially over 2600 Wh/kg! We cannot reach that full amount in real life. However, researchers and companies have already shown Li-S test batteries that store about 400-550 Wh/kg.
Compare this to today’s best Li-ion batteries in EVs. They typically store 150-260 Wh/kg. You can see the huge difference! What does this mean for you as a driver?
- Much Longer Range: Li-S batteries could double (or more) the energy a battery holds. This means EVs could travel much farther on one charge. Imagine EVs easily going over 500 or 600 miles. This would get rid of “range anxiety” for most trips.
- Lighter Vehicles: Car makers could also use Li-S batteries to get the same range as current EVs. But the battery pack would be much lighter. A lighter car uses less fuel, handles better, and speeds up faster.
Advantage 2: Lighter EVs, Better Performance
We mentioned this before, but saving weight is a big benefit. Lithium and sulfur are among the lightest elements used in batteries. Regular Li-ion batteries use heavier metals like cobalt, nickel, and manganese in their positive electrodes, and graphite in their negative electrodes.
Switching to the naturally lighter Li-S chemistry means the battery pack can lose much weight. The battery pack is sometimes the heaviest component of an electric vehicle. Experts who develop batteries for things like electric aircraft say that reducing weight is very important. You might not be flying an electric plane soon. However, the same idea applies to cars: a lighter EV simply works better and uses less energy to move.
Advantage 3: Potentially Lower Costs
Consider the materials. Sulfur is one of the most common elements on Earth. We often get it as a byproduct when refining fossil fuels. It is very cheap compared to cobalt and nickel. Cobalt and nickel are expensive, their prices change a lot, and only a few places in the world have them.
Using a lot of cheap sulfur for the main positive electrode material could greatly lower the cost of making batteries. Some companies, like Solidion Technology, even aim for future Li-S battery pack costs. They believe a 100 kWh pack (enough for about 500 miles of range, by their estimate) could cost the same as an internal combustion engine. These are future goals. However, the possibility of more affordable EVs, thanks to cheaper Li-S batteries, is a major reason for this research.
Advantage 4: More Environmentally Friendly?
Beyond cost, Li-S batteries offer possible environmental and ethical benefits. Mining cobalt, especially, has faced criticism. People are concerned about its impact on the environment and its link to bad working conditions in some areas. Reducing our need for cobalt by using sulfur is a big plus.
Some research also suggests that we might be able to make Li-S positive electrodes using more eco-friendly processes. This could even involve using water instead of the harsher, energy-intensive solvents often needed for Li-ion positive electrode production. We need a full study of its overall impact over its lifetime. However, the materials used in Li-S technology suggest it could be a greener battery.
The Challenges Facing Li-S Batteries
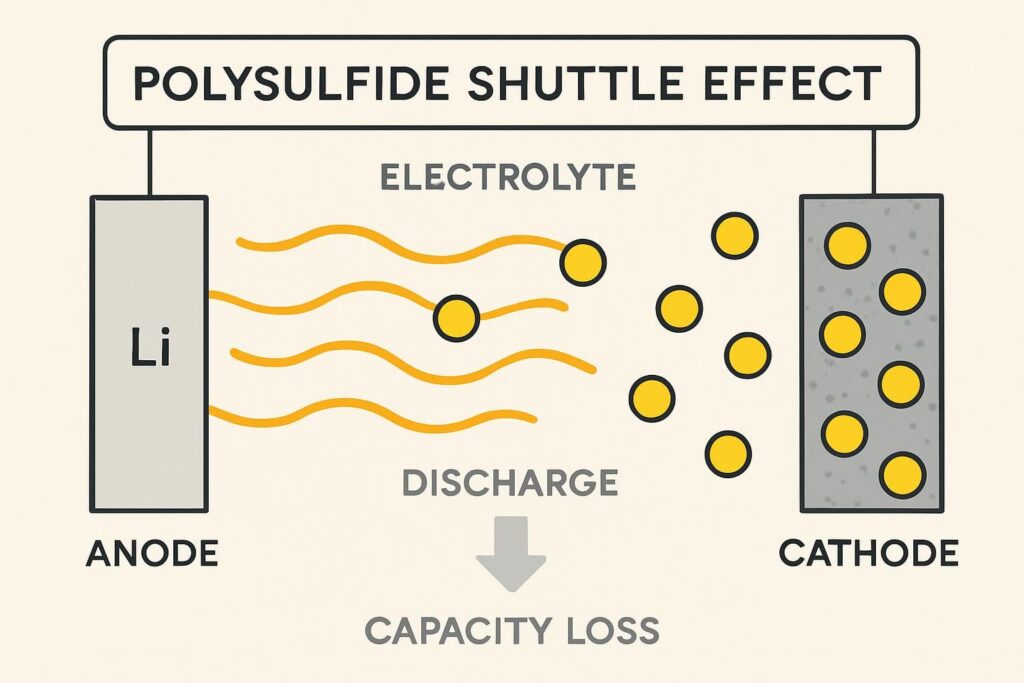
Remember those lithium polysulfides (Li2Sx) we talked about? They are the biggest problem for Lithium-Sulfur (Li-S) batteries. These sulfur compounds do not remain in the cathode. They easily dissolve into the liquid electrolyte. These sulfur compounds do not remain in the cathode.
1. Polysulfide Shuttle Problem
- Loss of Active Material: Sulfur that moves to the anode gets stuck there or reacts badly. This means it can no longer store energy at the cathode, causing the battery’s ability to hold charge to decrease quickly with each use.
- Low Efficiency: This back-and-forth movement uses up charge. Not all the electricity you put in during charging gets stored well.
- Anode Damage: Polysulfides react with the lithium anode. Pure sulfur is an insulator rather than a good conductor of electricity.
Because of this “shuttle effect,” Li-S batteries historically did not last very long. Users needed to replace them much sooner than lithium-ion batteries.
2. Sulfur’s Conductivity Issue & Volume Changes
Pure sulfur is an insulator rather than a good conductor of electricity. Manufacturers must mix in many conductive materials to make the cathode work, usually carbon. This adds weight and volume. These added materials do not store energy, reducing the actual energy density compared to the high theoretical value.
Also, when sulfur changes into lithium sulfide (Li2S) during discharge, its volume greatly increases—it swells by about 80%! This constant expanding and shrinking during charging and discharging puts stress on the cathode’s structure, causing it to crack and fall apart over time, further shortening the battery’s lifespan.
3. Lithium Metal Anode Woes (Dendrites)
Using pure lithium metal as the anode helps create high energy density. However, it also creates its own problems. When lithium returns to the anode during charging, it does not always spread smoothly. It can form tiny, needle-like, or mossy shapes called dendrites.
These Dendrites are Bad Because:
- Short Circuits: They can grow through the separator, which acts as a barrier between the anode and cathode. This can produce an internal short circuit, resulting in the battery overheating, failing, and possibly catching fire. This is a big safety concern.
- Efficiency Loss: Dendrite formation uses up lithium and electrolyte, reducing the battery’s capacity and efficiency over time.
4. Cycle Life – Making Them Last
Combine the shuttle effect, volume changes, and dendrite problems, and you can see why making Li-S batteries last a long time has been their biggest weakness. Cycle life is the number of times a battery may be charged and discharged before its capacity degrades considerably.
Lithium-ion batteries in electric cars can often last for thousands of cycles, which is enough for many people to drive for more than 10 years. But early Li-S batteries lost their charge much faster.
Scientists have made significant progress in labs. Some test batteries have shown 1,000, 2,000, or even 4,000 cycles under specific conditions. However, achieving this consistently in large, commercially ready batteries is still a major area of ongoing research and development. These batteries also need very few electrolytes. This is necessary for a high practical energy density.
Moving Forward: Big Steps And Recent Progress
Despite the difficulties, the great potential of Lithium-Sulfur (Li-S) batteries motivates researchers worldwide. They constantly invent and find smart solutions. The progress in recent years has been truly exciting!
Scientists are tackling the problems from many different angles:
- Smarter Cathodes: Researchers are creating advanced cathode structures, not just mixing sulfur with carbon. This includes:
- Carbon Hosts: They design porous carbon materials, like nanotubes or graphene. These materials can trap sulfur and polysulfides inside their structure. This helps limit the shuttle effect and improves how well they conduct electricity.
- Sulfurized Carbons (e.g., SPAN): Scientists are chemically attaching sulfur to polymer chains, such as polyacrylonitrile. This creates materials called SPAN that naturally stop polysulfides from dissolving. Companies like Zeta Energy are basing their technology on these cathodes that do not produce polysulfides.
- Metal Oxides/Sulfides/MOFs/LDHs: Researchers are adding materials that chemically connect with or trap polysulfides. This prevents them from moving back and forth and can also speed up chemical reactions.
- Protecting the Anode: To fight against dendrites and anode damage, researchers are working on:
- Artificial SEI Layers: They create stable, artificial protective layers on the surface of the lithium metal.
- Electrolyte Additives: These chemicals are added to the electrolyte. These chemicals help lithium spread more smoothly or form better natural protective layers. For example, researchers at Florida International University (FIU) reported using a sugar-based additive in 2021 to make batteries last longer.
- Advanced Separators: Scientists are developing separators with smaller holes or special coatings. An example is aramid nanofibers, which are like tiny Kevlar threads. Reported in 2022, these physically block dendrites or only allow lithium ions through while stopping polysulfides.
- Better Electrolytes: Researchers are designing new liquid electrolytes that do not dissolve polysulfides as easily. They are also exploring solid-state electrolytes. These could completely block polysulfide movement and potentially prevent dendrites, making the batteries safer.
- Functional Interlayers: Scientists add a unique layer between the cathode and the separator. This layer is designed to catch any stray polysulfides and help turn them back into useful forms.
- New Material Discoveries: Sometimes, breakthroughs come from unexpected places. In 2024, UC San Diego researchers revealed a new sulfur-iodine crystalline substance. It makes the cathode’s electrical conductivity much higher (by billions of times!). This material also has self-healing properties.
- Faster Charging: While not the main goal, some research aims to make Li-S batteries charge faster. For instance, DGIST in South Korea is using catalyst-doped materials. This could significantly reduce charging times.
These breakthroughs are not just theories. We are seeing real results:
- Researchers at Drexel University reported a Li-S battery chemistry in 2022. It showed no decline in performance over 4,000 charge cycles in lab tests. This was due to stabilising a specific form of sulfur.
- LG Chem, a major battery maker, showed a drone powered by its Li-S cells in 2020. This drone travelled across the stratosphere for 13 hours. LG Chem claimed these cells had an energy density of about 410 Wh/kg. They also stated plans for possible commercial production.
- New companies (startups) are actively working to bring these batteries to market. They are using specific innovations, like sulfurised carbon cathodes.
This continuous flow of new ideas shows the huge effort going into making Li-S batteries a practical reality.
Li-S vs. Li-ion: A Quick Comparison
| Feature | Lithium-Sulfur (Li-S) | Current Lithium-Ion (Li-ion) | Notes for EV Users |
|---|---|---|---|
| Energy Density (Wh/kg) | Very High (400–550+ practical) | Moderate (150–260) | Li-S promises much longer range or lighter vehicles. |
| Weight | Lighter | Heavier | Better vehicle efficiency and handling with Li-S. |
| Cost Potential | Lower (Abundant Sulfur) | Higher (Cobalt, Nickel) | Li-S could lead to more affordable EVs. |
| Cycle Life | Improving (Challenges remain) | Good (Well-established) | Li-ion currently lasts longer in real-world use. |
| Safety | Challenges (Li metal dendrites) | Generally Good (Mature Tech) | Li-S safety needs further development & validation. |
| Availability (TRL) | Emerging / Pre-commercial | Widely Commercial | Li-ion is what’s in EVs today; Li-S is coming soon. |
What Does This Mean For Your Next EV? The Road Ahead
Should you wait for Lithium-Sulfur (Li-S) batteries before buying an EV? Probably not. The progress is exciting, but we need to have realistic expectations. Lithium-sulfur technology is still in its advanced development and early commercialisation stages. You will not find Li-S batteries in new EVs at dealerships today.
Companies like LG Chem have mentioned possible production dates around 2025 or later. However, widespread use in cars usually takes more time. Manufacturers need to increase production, prove long-term reliability and safety in real driving conditions, and decrease costs to meet attractive price goals.
The journey from a promising lab result or a specialised drone flight to powering millions of cars is long and complex. Other new technologies, like solid-state batteries, also exist. These technologies have their own challenges and timelines.
However, the potential influence of Li-S batteries is clear. If Li-S technology successfully overcomes its remaining hurdles, we could see a future generation of EVs that:
- Offer driving ranges easily over 500 or 600 miles.
- They are lighter and more efficient.
- It could be much cheaper to buy.
Intense research and development continue. Li-S technology’s clear advantages drive this. It represents one of the most promising ways to address the main limits of current EV battery technology.
Conclusion
Lithium-sulfur batteries stand out as a great hope for better energy storage. They can greatly increase energy density compared to today’s Lithium-Ion batteries. They also use cheap, abundant sulfur. This provides a promising picture for the future of electric automobiles. This future includes much longer driving ranges and potentially lower prices.
Yes, big challenges remain. These include the polysulfide shuttle, dendrite formation, and achieving a long lifespan. But as we have seen, scientists and engineers are solving these problems with remarkable creativity. They are working on new materials and novel battery architectures. The progress is real. It is steadily bringing Li-S technology closer to practical applications.
You will not buy a Li-S-powered car tomorrow, but it is worth watching this technology. It provides a promising way to make EVs even more useful, affordable, and appealing to everyone. It truly unlocks the next stage of electric mobility.
FAQs
Are Lithium-Sulfur batteries available in EVs now?
No, not in mass-market electric cars. They are still in advanced development and pre-commercial stages. Some prototypes and specialised applications (like drones) have used them, but they are not yet widely available for automotive use.
How much longer range could Li-S batteries realistically provide?
Based on demonstrated prototype energy densities (400- 550+ Wh/kg compared to Li-ion’s 150-260 Wh/kg), Li-S batteries have the potential to offer significantly longer range, possibly 1.5 to 2 times (or even more) the range of current EVs with similar weight battery packs. This could mean future EVs with 500-600+ mile ranges.
Are Li-S batteries safer than Li-ion batteries?
Not necessarily, at least not yet. While sulfur is relatively safe, using highly reactive lithium metal as the anode presents challenges, particularly the risk of dendrite formation, which can cause short circuits and potential thermal runaway (fire). Significant research is focused on improving the safety of Li-S batteries before they can be widely adopted.
When can we expect to see Li-S batteries in commercial EVs?
Timelines are uncertain and depend on overcoming the remaining technical and manufacturing hurdles. Some optimistic projections from companies involved suggest initial commercial production might start around 2025 or later, but widespread adoption in mass-market cars would likely take several more years after that.
What are the main problems scientists need to solve for Li-S?
The key challenges include:
1) Suppression of the ‘polysulfide shuttle’ effect, which directly leads to capacity degradation.
2) Increasing the intrinsic electrical conductivity of the sulfur-based cathode.
3) Managing the large volume changes during charge/discharge.
4) Preventing lithium dendrite growth on the anode for safety and longevity.
5) Achieving long cycle life comparable to Li-ion under practical conditions.
Are Li-S batteries better for the environment?
Potentially, yes. They use abundant, cheap sulfur instead of ethically concerning and resource-limited cobalt or nickel found in many Li-ion batteries. Manufacturing might also be greener. However, a full lifecycle analysis considering lithium sourcing and end-of-life recycling is still needed for a complete picture.
How do Li-S batteries compare to solid-state batteries?
Both are considered promising next-generation technologies aiming for higher energy density and improved safety over conventional Li-ion. Solid-state batteries replace the liquid electrolyte with a solid material, which could inherently prevent dendrites and potentially enable lithium metal anodes. Li-S focuses on the high capacity of sulfur. Both face significant challenges in development and manufacturing scale-up, and it’s unclear which (if any) of these technologies will dominate the future market.







