Future of EV Battery Anodes: Silicon, Hybrids & More
EV battery anodes are essential components in electric vehicle batteries. They store energy during charging and release it during use. Traditionally made of graphite, anodes are evolving with advancements like silicon and hybrid materials, which promise to increase energy capacity, improve charging speeds, and extend battery life. These improvements are key to enhancing the performance of electric vehicles and supporting the industry’s shift towards more affordable, efficient EVs.
Understanding the Anode In Li-ion Battery
Think of your EV’s lithium-ion battery like a tiny hotel for energy particles called lithium ions. When you charge your car, these ions travel from one side of the battery (the cathode) to the other side, checking into the anode for storage. When you drive, they flow from the anode and travel back to the cathode, releasing energy to power your car. When the battery is charged, the side that retains those lithium ions is known as the anode, or negative electrode.
Your battery’s capacity to store energy increases with how well the anode accepts and retains these lithium ions. This directly results in more EV driving range. A good anode material must be like a well-organised parking garage—able to pack in lots of ions (high energy density) without taking up too much space or weight.
Furthermore, how quickly those ions can move into and out of the anode affects how fast you can charge your EV. An efficient anode allows for faster ion movement, contributing to the faster EV charging technology we all want. So, while it might seem like a small part, the anode is a true unsung hero, critical for your electric vehicle’s overall performance and convenience.
Graphite Anodes: The Current Standard and Its Limits
For a long time, graphite has been the king of anode materials in most lithium-ion batteries, including those in EVs. Why graphite? Well, it has several advantages. Its layered structure, like sheets of paper stacked together, provides neat little spaces for lithium ions to slide into and out of during charging and discharging. This makes it relatively stable and reliable over many charge cycles.
Graphite is also quite abundant and relatively inexpensive to produce, which has helped keep battery costs down and made EVs more accessible. Manufacturers have well-established processes for making graphite anodes, making it the practical choice for mass production. We’ve seen graphite anodes power millions of EVs reliably over the years.
However, like any reigning champion, graphite has its limitations. The biggest issue is that it’s nearing its theoretical limit for how much energy it can store. Think of that parking garage again – graphite’s structure means there’s a maximum number of ‘parking spots’ for lithium ions. We’re getting close to filling all those spots. We need materials that can hold more ions in the same space to boost EV range significantly.
Additionally, while graphite allows for decent charging speeds, pushing for ultra-fast charging can sometimes cause issues, like lithium metal plating on the anode surface, reducing battery life, and posing safety risks. This inherent property limits how quickly we can safely pump energy back into current EV batteries. Therefore, the quest for next-generation EV batteries with dramatically better performance inevitably leads us to explore materials beyond graphite. The need is clear: we require advanced lithium-ion battery materials to unlock the next level of EV capability.
The Silicon Anode Revolution: Packing More Power
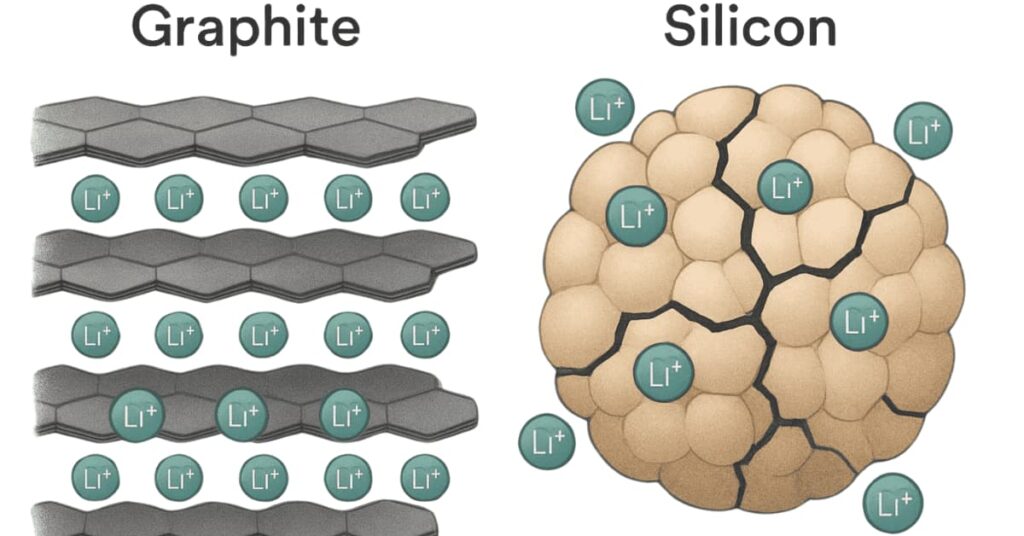
Silicon (Si) is an exciting material for battery anodes. It could theoretically hold ten times more lithium than graphite. Electric vehicle (EV) batteries could allow cars to travel much farther on one charge. We could see cars breaking the 500-mile range.
However, silicon has a big problem. It swells a lot (300-400%) when it charges, then shrinks when it discharges. This constant change breaks down the material over time, making batteries wear out quickly. Early silicon anodes did not last long enough for EVs.
Engineers and nanotech experts are working on solutions. They found that using large pieces of silicon doesn’t work. Instead, they design tiny silicon structures.
Key advancements in silicon anode technology include:
- Nanostructuring: This involves making tiny silicon structures. Examples include nanowires or porous silicon particles. These small forms have space to expand and contract without breaking. They also help lithium ions travel faster, which can improve charging speed.
- Silicon-Carbon Composites: Researchers mix small silicon particles with conductive carbon. The carbon acts as a flexible, conductive support, holding the silicon together during expansion and improving electrical connection.
- Using Silicon Oxides (SiOx): This method uses silicon suboxides instead of pure silicon. These materials expand less than pure silicon, balancing capacity and stability. Many commercial silicon anodes today use SiOx mixed with graphite.
Companies like Amprius Technologies are leading in this area. They make silicon nanowire anodes, which already offer much higher energy density than regular graphite anodes. This technology is useful for high-altitude satellites and future EVs. An industry report stated that silicon anode batteries show a breakthrough in performance. These batteries use silicon instead of graphite in their anodes.
Cost and mass production challenges still exist. However, progress is clear. Silicon anode batteries are moving from labs to mainstream EVs, promising a big jump in performance.
Summary: Silicon anodes offer high energy capacity for EV batteries but face challenges due to significant swelling and shrinking during charging/discharging, leading to degradation. Solutions involve nanostructuring, silicon-carbon composites, and silicon oxides to manage expansion and improve stability. Despite cost and manufacturing hurdles, companies like Amprius are advancing silicon anode technology for future EVs, promising improved range.
Hybrid and Composite Anodes: Making Batteries Better
Here’s the simplified content:
Scientists are exploring a new way to improve battery parts called anodes. Instead of using just one material, they mix different materials to create “hybrid” or “composite” anodes. This is like building a dream team: each material brings its own strengths, making the new material perform better than any single one.
For example, Professor Jae-Min Oh and Professor Seung-Min Paek created a new material. They combined a super-conductive material called reduced graphene oxide (rGO) with nickel-iron compounds (NiFe₂O₄ and NiO). The rGO helps electrons move very fast. The nickel-iron parts store energy quickly through a process called pseudocapacitance, which is faster than how graphite works. They built this material using a special stacking method to make hollow spheres.
Their new anode material performed very well. It stored 1687.6 mA h g⁻¹ of energy even after 580 cycles. This is much better than traditional graphite. Professor Jae-Min Oh believes that future energy storage materials will use multiple interacting materials to create a combined effect, leading to smaller, lighter, and more efficient energy storage. This research shows how carefully combining materials at a tiny level can greatly improve battery performance.
Another exciting area is Tin-Hard Carbon Anodes. Tin can store more lithium than graphite, but also expands a lot. Scientists are putting tiny tin particles inside a strong material called hard carbon. The hard carbon supports the tin and helps it conduct electricity, which reduces the expansion problem and makes the battery last longer. These combinations also offer a way to create anodes that store more energy.
These examples show that the best anodes for the future might come from cleverly combining different materials. This approach helps create a good balance of energy storage, power, lifespan, and safety.
Summary: Hybrid and composite anodes combine different materials for superior battery performance. One example is rGO and nickel-iron compounds, which offer excellent conductivity and fast energy storage, leading to higher capacity and stability. Another promising approach involves encapsulating tin nanodots within hard carbon to manage expansion issues and improve cycle life. The future of anodes likely involves intelligently combining materials for optimal energy density, power, lifespan, and safety.
Exploring New Battery Materials: Anodes For Future EVs
Scientists are looking into advanced materials for the next generation of electric vehicle (EV) batteries. These materials may not be ready for EVs right away. But they show great promise for the future.
- Lithium Metal Anodes: Pure lithium metal is seen as the ideal anode. It can store the most energy. However, it has big problems. When charging, sharp, needle-like growths called dendrites form. These dendrites can poke through the battery’s separator, causing short circuits and even fires. A lot of research focuses on making lithium metal safe. This research often involves solid-state batteries, which use a solid material instead of a liquid to stop dendrites.
- Graphene in Battery Anodes: Graphene is a single layer of carbon atoms. It is very strong, light, and conducts electricity well. It can improve silicon or other anode materials. It might even be a main part of an anode. Its ability to conduct electricity could make EV charging faster. Its strength could also make batteries last longer. But making good graphene cheaply and in large amounts is still hard.
Researchers are also studying other materials, including various metal oxides and different types of engineered carbons. The search for new anode materials is very active. Scientists are always trying new chemical mixes and structures to improve how we store energy.
What Really Matters? Key Metrics for Advanced Anodes
With all these different materials and technologies being developed, how do we compare them? What makes a ‘good’ anode for an EV battery? Here are the key performance indicators (KPIs) that researchers and manufacturers focus on:
Energy Density (measured in mAh/g or Wh/kg): This is arguably the most talked-about metric. It tells you how much energy the anode material can store per unit of weight or volume. Higher energy density means a longer EV driving range for the same size battery pack, or a lighter battery pack for the same range.
Rate Performance (Charging Speed): How quickly can the anode accept lithium ions during charging? This determines how fast you can recharge your EV. Materials with good rate capability allow for faster EV charging technology without causing damage or significant performance loss.
Cycle Life: How many times can the battery be charged and discharged before its capacity drops significantly (e.g., below 80% of its original capacity)? A long cycle life is crucial for battery longevity, ensuring your EV battery lasts for many years and miles. Extending battery cycle life is a major goal.
Safety: This is paramount. Anode materials must be stable and resistant to issues like thermal runaway (overheating) or dendrite formation (in the case of lithium metal). Enhancing battery safety is non-negotiable.
Cost and Scalability: A fantastic new material is useless if it’s prohibitively expensive or impossible to manufacture at the massive scale needed for the EV industry. Reducing battery manufacturing costs and ensuring materials can be produced reliably in large quantities are critical for market adoption.
Sustainability: Where do the raw materials come from? Are they ethically sourced? Can they be recycled? Sustainable battery materials sourcing and end-of-life recycling are becoming increasingly important considerations.
Finding the perfect anode material involves balancing all these factors. Often, improving one metric might negatively impact another (e.g., increasing energy density might reduce cycle life or safety). The goal is to find the optimal trade-offs for specific applications, especially for demanding ones like electric vehicles.
Comparison Of Anode Materials For Li-ion EV Batteries
| Material | Theoretical Capacity (mAh/g) | Practical Capacity (mAh/g) | Cycle Life | Charging Speed | Key Advantages | Key Challenges |
|---|---|---|---|---|---|---|
| Graphite (Current Standard) | ~372 | ~330-360 | Good (1000s) | Moderate | Stable, low cost, mature technology | Reaching energy density limit, moderate speed |
| Silicon (Si) | ~4200 | >1000 (Target) | Poor (Raw) | Moderate-Fast | Very high energy density potential | Massive volume expansion, poor cycle life (raw) |
| Silicon Composites (Si-C/SiOx) | 1000-2000+ | ~400-1000+ | Moderate-Good | Moderate-Fast | Higher energy density than graphite, improved stability | Volume expansion still a challenge, cost, complexity |
| Hybrid (e.g., rGO/NiFe₂O₄) | Variable (High) | ~1600+ (Example) | Good (Reported) | Potentially Fast | High capacity, good stability (engineered) | Complexity, scalability, cost |
| Tin-Carbon (Sn-C) | ~990 (Sn) | Moderate-High | Moderate | Moderate | Higher capacity than graphite | Volume expansion, cycle life |
| Lithium Metal (Li) | ~3860 | Very High | Poor (Dendrites) | Potentially Fast | Highest theoretical energy density | Dendrite growth, safety risks, reactivity |
Overcoming Hurdles In Anode Development
We’re not quite there yet, even though the future appears bright. Several major obstacles must be overcome to get these cutting-edge anode materials from the lab into millions of EVs on the road:
Scaling Up Production: Making small amounts of a novel material in a lab is one thing; producing it consistently, reliably, and cost-effectively at the massive scale required by the automotive industry (terawatt-hours worth of batteries) is a monumental challenge. New manufacturing processes need to be developed and refined.
Cost: Many advanced materials and nanostructuring techniques are currently expensive. Reducing battery manufacturing costs associated with these new anodes is crucial for making next-generation EVs affordable for the average consumer.
Long-Term Durability and Safety: While lab tests are promising, these new anodes need to prove their stability, safety, and longevity over many years and hundreds of thousands of miles in real-world driving conditions, experiencing vibrations, temperature fluctuations, and varying charge/discharge patterns.
Supply Chain Development: Sourcing the raw materials for some of these new anodes (like silicon or specific metals for composites) sustainably and in sufficient quantities requires building robust and ethical supply chains.
Integration: New anode materials often need to work seamlessly with other battery components (cathode, electrolyte, separator). Integrating them into existing battery cell designs and manufacturing lines can require significant adjustments and re-engineering.
Researchers and battery manufacturers are actively working on solving these challenges. It requires collaboration across materials science, chemical engineering, manufacturing, and supply chain management. Progress is being made rapidly, but it will take time and continued investment to realize the potential of these advanced anodes fully.
Conclusion
We stand at an exciting crossroads in the evolution of electric vehicles. The humble anode, once a relatively straightforward component, has become a major focus of innovation, driving the quest for better lithium-ion EV batteries. While graphite has served us well, its limitations are pushing us towards revolutionary new materials.
The silicon anode revolution promises a dramatic leap in energy density, potentially banishing range anxiety for good. Meanwhile, hybrid and composite anodes showcase the power of clever material engineering, combining different strengths to achieve superior all-around performance. And further out, frontiers like lithium metal and advanced graphene concepts hint at even greater possibilities.
These advances in anode materials aren’t just about technical specifications; they’re about fundamentally improving the EV experience for everyone. They pave the way for cars that go further, charge faster, last longer, and become more affordable and sustainable. The journey involves overcoming significant manufacturing and cost hurdles, but the momentum is undeniable.
As someone deeply interested in this technology, I can tell you that the pace of anode material research and development is staggering. The silent revolution within the battery is getting louder, and it’s charging us towards a future where electric driving is not just viable, but vastly superior. Keep an eye on this space – the next big breakthrough might be just around the corner, ready to power your drive.
Frequently Asked Questions (FAQs)
What exactly is an anode in an EV battery?
Think of an anode as the energy storage part of your EV’s lithium-ion battery when it’s charged. This part, usually made of graphite, soaks up lithium ions during charging. It then releases them when you drive, giving your car power.
Why is graphite the standard anode material now, and what’s wrong with it?
Graphite is common because it’s stable, cheap, and easy to make. It has worked well for many years. However, it can only store a limited amount of energy, which limits how far EVs can go and restricts how fast you can safely charge the battery.
Why Are Silicon Anodes Exciting?
Silicon is exciting because it can hold about 10 times more lithium ions than graphite in the same space! This could mean much longer driving ranges for EVs. Or, it could mean lighter batteries for the same range.
If Silicon is So Good, Why Isn’t It in All EVs Yet?
The main problem is that silicon swells a lot (300-400%) when it takes in lithium ions. It then shrinks when it releases them. This constant change breaks down the silicon quickly, shortening the battery’s life. Scientists are creating smart, tiny structures and mixtures (like silicon with carbon). These help manage the swelling and make silicon anodes last longer.
Are silicon anodes actually being used in EVs today?
Yes, but usually not pure silicon. Many advanced batteries today use a mix. They often add a small amount of silicon (like silicon oxide or in a mixture) to graphite. This boosts energy while still keeping the battery stable. Companies like Amprius make high-energy silicon anodes. These are first for special uses. But the technology is moving towards regular EVs.
What are hybrid or composite anodes?
These anodes combine different materials, using the best parts of each. For example, they might mix conductive graphene with metal oxides that can hold a lot of energy. Or, they might put tin inside carbon. The goal is to make a new material that works better overall, including capacity, stability, and charging speed. It performs better than any single material alone.
Will these advanced anode materials make EVs cheaper?
Not necessarily at first. Making these new materials can be more complicated and expensive than using graphite. But in the long run, higher energy density could mean smaller battery packs are needed for the same range. This might lower the total cost of the battery pack. Also, as more are made, costs should drop.
Are these new anode materials safe?
Safety is a top concern. Materials like pure silicon or lithium metal have challenges like swelling, but much research focuses on designing solutions to keep them stable. The goal is to prevent safety issues like overheating. Any new material must pass strict safety tests before being used in commercial EVs.