Understanding the Charging Speed of Solid-State Batteries
During the charging of solid-state batteries, electrons travel between the positive electrode (cathode) and the negative electrode (anode). The battery stores more chemical potential energy when the electric charge flows in a specific manner.
This stored energy transforms into electricity when the battery powers a device. The battery’s electrical charge is maintained by the flow of ions, which is facilitated by a solid substance known as the electrolyte. This solid electrolyte, often made of materials such as lithium phosphorus oxynitride (LiPON), makes the battery safer, particularly when it gets very hot, a condition that can increase the risk of ignition in lithium-ion batteries.
Solid-state batteries use the electrolyte as a separator, which keeps the positive and negative parts apart, leading to better stability and the ability to be recharged multiple times. In contrast, lithium-ion batteries require a distinct separator.
Do solid-state batteries charge faster?
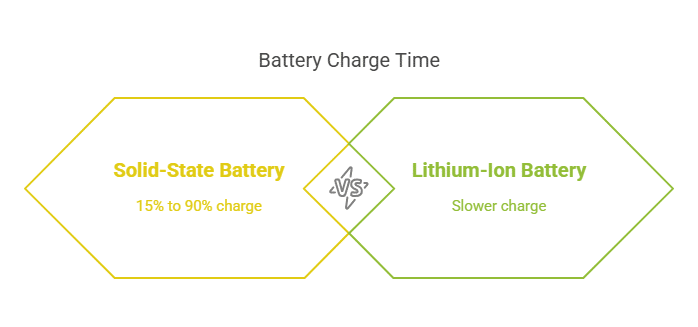
Solid-state batteries could significantly change electric vehicles because they charge faster than regular lithium-ion batteries. Traditional lithium-ion batteries use liquid to move energy, but solid-state batteries use a solid material for more efficient energy movement between the battery parts. The different design allows solid-state batteries to charge more quickly; some can charge from 15% to 90% in just 18 minutes at room temperature.

Using materials that hold more energy, like lithium metal, helps them charge rapidly without reducing the car’s range or battery power. As more people want solid-state batteries, companies such as Stellantis and Factorial are improving battery performance with technologies like Factorial Electrolyte System Technology (FEST), which helps batteries charge faster and work better overall.
These improvements suggest that solid-state batteries will charge electric vehicles more quickly and last longer, making them a promising development for the future of electric transportation.
Components Of Solid-State Batteries
To understand the charging process, let’s first take a quick look at the core components of a solid-state battery:
Anode: An anode is the electrode that receives an electrical charge during charging. Solid-state batteries are composed of lithium and other materials that hold ions.
Cathode: The cathode acts as an electrode. Electric discharge happens through the cathode. Metal oxides and other materials form the cathode. These materials accept lithium ions and release them.
Solid Electrolyte: Solid-state batteries use a solid electrolyte material, replacing the traditional liquid electrolyte. The solid electrolyte prevents the anode and cathode from creating a short circuit within the battery. Lithium ions move through the solid electrolyte during the battery’s charge and discharge cycles.
Separator: The separator is a thin layer of non-conductive material that prevents direct contact between the positive and negative parts.
In summary, the anode, cathode, solid electrolyte, and separator are critical components of solid-state batteries, each playing a unique role in the battery’s overall function and efficiency. Understanding these components is key to appreciating the advancements in battery technology and their potential applications.
The Process Of Charging Solid-State Batteries
Charging a solid-state battery is similar to charging other types of batteries, but it has some critical differences.
1. Energy Input Via Charging Source
A solid-state battery also requires an energy source for charging. Solid-state energy usually comes from a wall charger, although it can also come from a wireless pad if the system is compatible with it. The charger modifies the power so that the battery can use it effectively.
2. Flow of Ions And Electrons
The charger initiates charging by sending electrical pressure to the battery. Electrical pressure forces electrons to move. Electrons travel from the positive side of the battery to the negative side. They move outside the battery through wires. Simultaneously, ions move inside the battery, traveling through a solid electrolyte.
Solid-state batteries utilize a primarily solid electrolyte, which allows ions to travel within the battery cell. The batteries use components made mainly of ceramic or polymer materials that facilitate the movement of ions. This process helps improve the battery’s energy efficiency.
3. Charging At The Anode
Lithium ions move within the battery, and electrons flow to the outside circuits. The flow of electrons powers your device. When you plug in your device, the power supply sends current into the battery. The current reaches the lithium ions at the anode. The external circuit ensures the energy is used correctly.
4. Electrons Return To The Cathode
Electrons in the outside circuit move toward the cathode, the positive terminal. When they reach the cathode, they neutralize the battery’s charge. The electrochemical reaction at the cathode stores energy and charges the battery effectively.
5. Voltage And Current Management
The voltage and current are carefully controlled during the charging cycle to prevent damage to the battery. In a solid-state battery, the solid electrolyte manages this process with greater stability than liquid electrolytes, which can become unstable under high voltage. This stability helps prevent overheating and reduces battery degradation during charging.
6. Full Charge Completion
When the battery reaches a complete charge, the charger halts the current flow, and the battery stores its maximum energy at that moment. Unlike regular lithium-ion batteries, solid-state batteries generally avoid problems like capacity loss and the memory effect. Therefore, they preserve performance across numerous charging cycles.
How To Safely Charge Solid-State Batteries
Adhering to specific guidelines is crucial for charging solid-state batteries safely, which increases their expected lifespan and ensures maximum productivity. Here are some valuable suggestions for better charging practices for your battery:
Avoid Overcharging: It’s always advisable to shut down the device after the battery is fully charged. Otherwise, overcharging may occur, which can be detrimental to battery life.
Keep It Cool: To keep the battery cool, charge the solid-state device in a calm, dry environment. Never charge the device in direct sunlight or hot areas, as heat can damage the battery.
Use the Right Charger: Always use a specialized charger for your solid-state battery. It will ensure that the battery charges at the appropriate speed without overheating.
Charge Regularly: To maintain a healthy, solid-state battery, ensure it is not completely discharged before charging it again. Regular charging helps sustain its performance and increase its longevity.
Benefits Of Charging Solid-State Batteries
In comparison with conventional lithium-ion batteries, charging solid-state batteries offers several benefits:
Faster charging times: Solid-state batteries can charge more quickly and efficiently by using a solid electrolyte instead of a liquid electrolyte.
Increased energy efficiency: Solid-state batteries are more energy-efficient as they can withstand higher charge currents without significant degradation.
Better thermal management: Solid electrolytes conduct heat more efficiently, making solid-state batteries less likely to overheat during charging.
Longer lifespan: A solid-state battery lasts longer as it experiences lower wear and tear during charging processes.
Conclusion
Solid-state batteries transform energy storage, offering quicker charging and improved safety compared to traditional lithium-ion batteries. Traditional lithium-ion batteries use liquid substances to transfer energy; however, solid-state batteries utilise a solid substance, enabling more effective energy transfer and resulting in faster charge times.
Solid-state batteries can gain charge from 15% to 90% in just 18 minutes at normal temperatures due to sophisticated materials, such as lithium metal. The creation guarantees swifter charging and preserves a vehicle’s driving distance and battery capacity.
Corporations such as Stellantis and Factorial are propelling this progress with technologies like Factorial Electrolyte System Technology (FEST), which improves both speed and effectiveness. These developments establish solid-state batteries as a promising solution for electric vehicles, delivering faster charging, extended durability, and improved overall performance.